What is the Process for Establishing changhong yun oligo lc-ms Bioanalytical Methods?
Liquid chromatography-mass spectrometry (LC-MS) is a powerful analytical technique widely used in the biopharmaceutical industry, particularly for the quantitative analysis of biomolecules such as oligonucleotides. The development of sensitive and specific LC-MS/MS methods is crucial for the successful characterization and quantification of oligonucleotides in various biological matrices, including plasma, serum, and tissue.

This article outlines the key steps involved in establishing a robust LC-MS/MS bioanalytical method for oligonucleotides, drawing on our experience and industry best practices.
Key Steps in LC-MS/MS Method Development
Here are the key steps of changhong yun oligo lc-ms development:
Optimization of MS Conditions
Accurately determining the charge state distribution of an oligonucleotide is crucial for optimizing MS parameters, with factors like sequence, solvent composition, and instrument settings influencing the results. Electrospray ionization (ESI) is commonly used for ionization, and understanding the charge state distribution can improve analysis.
Mobile phase optimization is also essential to minimize metal adduct formation, which can degrade sensitivity and selectivity; ammonium acetate or ammonium formate buffers are often employed to suppress this issue. Finally, optimizing MRM transitions by selecting the right precursor and product ions enhances the sensitivity and reliability of quantitative mass spectrometry analysis.
Optimization of Chromatographic Conditions
Column selection plays a vital role in oligonucleotide separation, with reversed-phase columns, typically using C18 or C18-like stationary phases, being the most common. Factors like column length, particle size, and pore size can impact separation efficiency and peak shape.
Mobile phase optimization, including the balance of organic solvent and buffer concentration, is crucial for controlling retention time, peak shape, and ionization efficiency, with gradient elution often used for optimal separation. Temperature control also enhances resolution and peak shape, where lower temperatures improve retention, and higher temperatures increase peak capacity and reduce analysis time.
Optimization of Sample Storage Conditions
Container selection is crucial to prevent analyte adsorption and degradation, with low-binding polypropylene or glass tubes being commonly used. Choosing the right solvent, such as aqueous solutions with acetonitrile or methanol, helps maintain the stability of oligonucleotide samples. Temperature control is equally important to preserve sample integrity, with storage typically optimized at -80°C to minimize degradation. Proper container, solvent, and temperature conditions are essential for ensuring the long-term stability of oligonucleotide samples.
Optimization of Sample Extraction Conditions
Efficient extraction of oligonucleotides from biological matrices is crucial for accurate quantification, with solid-phase extraction (SPE) and liquid-liquid extraction (LLE) being widely used methods. The stability of the oligonucleotides during extraction, including steps like drying and reconstitution, must be carefully evaluated to minimize sample loss and degradation. Ensuring stable conditions throughout the process is essential for maintaining sample integrity. Proper extraction and stability measures are key for reliable analysis and quantification.
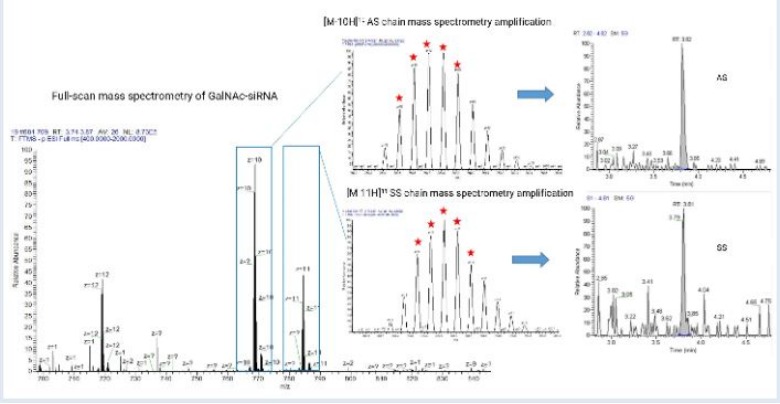
Method Qualification
The method should be sensitive enough to accurately measure the analyte at the lower limit of quantification (LLOQ). It must also be selective, minimizing interference from other components or analytes in the sample matrix. Reproducibility is essential, with precise and accurate results across multiple tests. Additionally, matrix effects should be evaluated and minimized through proper sample preparation and the use of internal standards, while sample stability in various conditions ensures consistent, reliable outcomes.
Conclusion
The development of robust and sensitive LC-MS/MS bioanalytical methods for oligonucleotides is essential for supporting drug discovery, development, and clinical studies. By following the key steps of changhong yun oligo lc-ms outlined in this article, researchers can develop high-quality methods that meet the rigorous standards required for regulatory submissions.
