Hemolysis Labs Testing: The Detailed Guide about Causes, Effects, and Mitigation Strategies
Hemolysis, the rupturing of red blood cells (RBCs) during blood sample processing, is a prevalent preanalytical concern that can significantly impact the accuracy and reliability of laboratory test results. This article explores the various causes of hemolysis, its detrimental effects on bioanalysis, and effective tactics for minimizing its occurrence.

Causes of Hemolysis in Laboratory Samples
Hemolysis can occur due to a variety of preanalytical and analytical factors. Physiological conditions, such as anemia, dehydration, or electrolyte imbalances, can make red blood cells (RBCs) more fragile and prone to hemolysis. Additionally, improper phlebotomy practices, including using a small needle, applying excessive tourniquet pressure, or shaking the sample tube vigorously, can mechanically rupture RBCs.
Delays in sample processing, storage at inappropriate temperatures (either too hot or too cold), and excessive centrifugation can also lead to RBC damage. Analytical causes of hemolysis include freeze-thaw cycles, where repeated freezing and thawing of blood samples weaken the RBC membranes and promote hemolysis. Furthermore, certain laboratory assays may be particularly sensitive to hemolyzed samples, potentially resulting in inaccurate test results.
Effects of Hemolysis on Bioanalysis
The released intracellular components from lysed RBCs, primarily hemoglobin, can interfere with various analytical processes, compromising test results in several ways:
● Matrix effects: Hemoglobin can bind to analytes of interest or analytical reagents, altering their detection or quantification.
● Extraction recovery: Hemoglobin can impede the extraction efficiency of target analytes from the sample matrix.
● Analyte stability: Hemoglobin can act as a catalyst for analyte degradation, reducing their measured concentrations.
● Impact on specific analytes: Hemoglobin's spectral properties can interfere with the measurement of analytes that share similar absorption characteristics. This is particularly concerning for analytes with a high blood-to-plasma concentration ratio or those encapsulated in liposomes.
The International Council for Harmonisation (ICH) guidelines, specifically ICH M10 on bioanalytical method validation, emphasize the importance of assessing the impact of hemolysis on analytical methods. This ensures the selectivity and accuracy of test results in the presence of hemolyzed samples.
Strategies to Minimize Hemolysis
Implementing a comprehensive strategy to minimize hemolysis is crucial for maintaining the integrity of laboratory testing. Here are some key tactics:
● Standardized phlebotomy practices: Well-trained phlebotomists using appropriate needle sizes, minimizing tourniquet time, and employing gentle blood collection techniques can significantly reduce hemolysis.
● Proper sample handling: Prompt processing of blood samples after collection, adhering to recommended storage temperatures, and avoiding excessive centrifugation are essential to prevent hemolysis.
● Visual inspection for hemolysis: Laboratory personnel should visually inspect samples for hemolysis upon arrival. Moderately or severely hemolyzed samples may require re-collection or alternative testing methods.
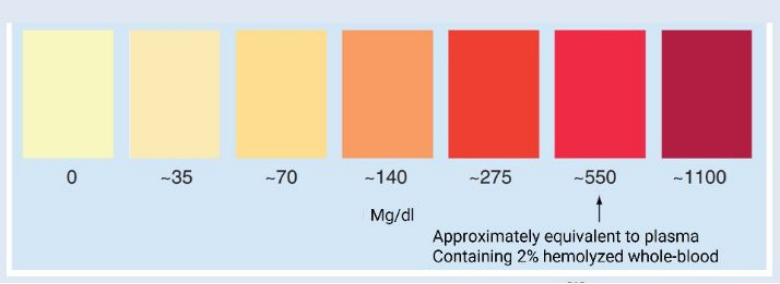
● Centrifugation protocols: Following established centrifugation protocols that consider factors like sample type, g-force, and centrifugation time can minimize hemolysis.
● Validated analytical methods: Employing analytical methods validated for their performance in the presence of hemolysis can help ensure the reliability of test results from hemolyzed samples.
Conclusion
Hemolysis remains a significant challenge in laboratory testing. By understanding the causes and effects of hemolysis, and by implementing effective strategies for its prevention and mitigation, hemolysis labs can ensure the accuracy and reliability of their test results, ultimately leading to improved patient care.
